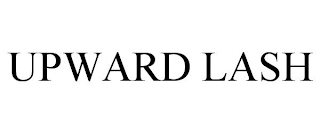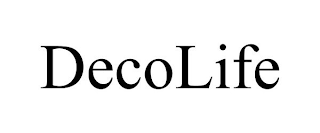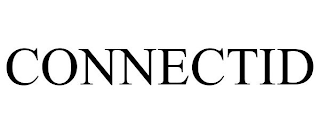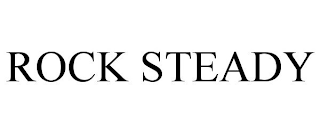BROWSE US TRADEMARKS BY CALENDAR
Search
Trademarks filed on Jun 02, 2023 (Total 1725 trademarks)
DRAIVER
Filed on Jun 02, 2023
Downloadable mobile application using artificial intelligence and machine learning for vehicle delivery logistics to manage trip itineraries, move vehicles by planning, executing, and instructing drivers, and moving freight and/or goods
DriverDo LLC
Suite 141,7900 College Boulevard, Overland Park, KANSAS, UNITED STATES, 66210
Suite 141,7900 College Boulevard, Overland Park, KANSAS, UNITED STATES, 66210
HANNVIW
Filed on Jun 02, 2023
Controllers for toy cars, planes; Electronically operated toy motor vehicles; Infant toys; Puzzle board games; Radio controlled toy cars, race cars, airplanes, boats; Remote-controlled toy planes; Remote control toys, namely, cars, race cars, airplanes, boats; Rideable toy vehicles; Scale model kits; Toy aircraft; Toy cars; Toy drones; Toy model cars; Toy models; Toy robots
Guangzhou Miaoyunhan Technology Co., Ltd.
Room CO119, 4th Floor, No. 190,Xianlie East, Tianhe District, Guangzhou, CHINA, 510000
Room CO119, 4th Floor, No. 190,Xianlie East, Tianhe District, Guangzhou, CHINA, 510000
ROCK STEADY
Filed on Jun 02, 2023
Cases for mobile phones; Cases for smartphones; Cell phone cases; Protective cases for cell phones; Protective cases for smartphones; Waterproof cases for smart phones
GULF BREEZE IP LLC
11665 COLLIER BLVD. #909, NAPLES, FLORIDA, UNITED STATES, 34116
11665 COLLIER BLVD. #909, NAPLES, FLORIDA, UNITED STATES, 34116
BLACK WOMEN IN NAILS
Filed on Jun 02, 2023
(Based on Use in Commerce) On-line customer-based social media brand marketing services; Providing business information in the field of social media(Based on Intent to Use) Providing a live forum for companies to showcase, display, demonstrate and promote new and innovative ideas, products and services in the convention/meeting management arena
Trammell, Taylia
2008 evergreen dr se, Conyers, GEORGIA, UNITED STATES, 30013
2008 evergreen dr se, Conyers, GEORGIA, UNITED STATES, 30013
SENSE
Filed on Jun 02, 2023
Gloves, namely, nitrile gloves and disposable gloves
Stauffer Manufacturing, Inc.
361 E. 6Th Street, Red Hill, PENNSYLVANIA, UNITED STATES, 18076
361 E. 6Th Street, Red Hill, PENNSYLVANIA, UNITED STATES, 18076
UPWARD LASH
Filed on Jun 02, 2023
Cosmetics
Make-Up Art Cosmetics Inc.
767 Fifth Avenue, New York, NEW YORK, UNITED STATES, 10153
767 Fifth Avenue, New York, NEW YORK, UNITED STATES, 10153
SLIMYGLOOP BUTTERGLOOP
Filed on Jun 02, 2023
Children's activity kits sold as a unit for making toy modeling dough in the nature of slime; toy modeling compounds
Horizon Group USA Inc.
430 Mountain Ave., Suite 205, New Providence, NEW JERSEY, UNITED STATES, 07974
430 Mountain Ave., Suite 205, New Providence, NEW JERSEY, UNITED STATES, 07974
TELAVANT
Filed on Jun 02, 2023
Pharmaceutical preparations for human use; biologic preparations for medical and therapeutic purposes; pharmaceutical preparations to treat gastrointestinal diseases and disorders and immunological diseases and disorders; biologic preparations to treat gastrointestinal diseases and disorders and immunological diseases and disorders
TELAVANT HOLDINGS, INC.
1 DNA WAY, SOUTH SAN FRANCISCO, CALIFORNIA, UNITED STATES, 94080
1 DNA WAY, SOUTH SAN FRANCISCO, CALIFORNIA, UNITED STATES, 94080
TRAVEL SLEEPER
Filed on Jun 02, 2023
I developed and sell a Self-Inflating mattress that is used for SUV car camping and road trips, for resting and sleeping. The mattress fits inside most SUV main trunks
Jones, Gerald
864 Stradford Circle, Buffalo Grove, ILLINOIS, UNITED STATES, 60089
864 Stradford Circle, Buffalo Grove, ILLINOIS, UNITED STATES, 60089
DECOLIFE
Filed on Jun 02, 2023
Hammocks; Tents; Awnings for vehicles of textile or synthetic materials; Bags for securing valuables; Drop cloths; Fabric and polyester mesh net used for storing toys and other household items; Fabric cabanas; Garment bags for storage; Laundry wash bags; Mesh bags for washing laundry; Pet hammocks; Portable toy storage bag; Rope for use in pet toys; Shoe bags for storage; Waterproof bags, namely, wet bags for temporary storage of wet and/or soiled cloth diapers
Guangzhou Dekelai Trading Co., Ltd.
Room M222, Room 301-353, No. 1,Kehua Street, Tianhe District, Guangzhou, CHINA, 510000
Room M222, Room 301-353, No. 1,Kehua Street, Tianhe District, Guangzhou, CHINA, 510000
SCHULER
Filed on Jun 02, 2023
Cabinets
ACPI WOOD PRODUCTS, LLC
20000 VICTOR PKWY, LIVONIA, MICHIGAN, UNITED STATES, 48152
20000 VICTOR PKWY, LIVONIA, MICHIGAN, UNITED STATES, 48152
VIRTUAL BINDER
Filed on Jun 02, 2023
Providing non-downloadable online case management software to collect and manage information, assign and track work and generate automated documents, in the field of employment law and employment litigation
Littler Mendelson, P.C.
333 Bush Street, 34th Floor, San Francisco, CALIFORNIA, UNITED STATES, 94104
333 Bush Street, 34th Floor, San Francisco, CALIFORNIA, UNITED STATES, 94104
TMMD
Filed on Jun 02, 2023
Operating on-line marketplaces for sellers and buyers of goods and/or services
MOUCKY LLC
50 industrial cir #105, Lincoln, RHODE ISLAND, UNITED STATES, 02865
50 industrial cir #105, Lincoln, RHODE ISLAND, UNITED STATES, 02865
WARRIOR FISHING
Filed on Jun 02, 2023
Organizing, arranging, and conducting fishing events
Take a Warrior Fishing, Inc
1021 Jensen Grove Ct, Fuquay Varina, NORTH CAROLINA, UNITED STATES, 27526
1021 Jensen Grove Ct, Fuquay Varina, NORTH CAROLINA, UNITED STATES, 27526
CONNECTID
Filed on Jun 02, 2023
Construction consultancy; construction of civil engineering structures; construction administration services, namely, construction project management services, construction supervision, and construction management
Walter P. Moore and Associates, Inc.
Suite 1100,1301 McKinney, Houston, TEXAS, UNITED STATES, 77010
Suite 1100,1301 McKinney, Houston, TEXAS, UNITED STATES, 77010
ROCK STEADY
Filed on Jun 02, 2023
Cases for mobile phones; Cases for smartphones; Cell phone cases; Protective cases for cell phones; Protective cases for smartphones; Waterproof cases for smart phones
GULF BREEZE IP LLC
11665 COLLIER BLVD. #909, NAPLES, FLORIDA, UNITED STATES, 34116
11665 COLLIER BLVD. #909, NAPLES, FLORIDA, UNITED STATES, 34116
RISING SUN JEWELRY
Filed on Jun 02, 2023
Jewelry
Sarah R Hunter
PO Box 7072, Auburn, CALIFORNIA, UNITED STATES, 95604
PO Box 7072, Auburn, CALIFORNIA, UNITED STATES, 95604
DOWNTOWN
Filed on Jun 02, 2023
party games
MONOLITH BOARD GAMES
153 Boulevard Haussmann, Paris FRANCE 75008
153 Boulevard Haussmann, Paris FRANCE 75008
AIRFLOWCARE
Filed on Jun 02, 2023
portable device for personal use for the treatment of light health problems, controllable via mobile application
Krupa, Ondrej
Vrchni cesta 122, Stramberk CZECHIA 74266
Vrchni cesta 122, Stramberk CZECHIA 74266
OLIVIA'S ORGANICS
Filed on Jun 02, 2023
Organic baby food
State Garden, Inc.
240 Second Street, Chelsea, MASSACHUSETTS, UNITED STATES, 02150
240 Second Street, Chelsea, MASSACHUSETTS, UNITED STATES, 02150
EXPERT ADVICE. SMART SOLUTIONS. COMPLIANCE SUCCESS.
Filed on Jun 02, 2023
Business consulting services for life sciences, pharmaceuticals, biotechnology and medical device companies in the nature of regulatory compliance program development and enhancement and transparency program support, namely, reviewing program status and performing gap analyses, drafting programs plans, program implementation support, development and improvement of policies, standard operating procedures and codes of conduct; business consulting services in the nature of monitoring of pharmaceutical and medical device manufacturer interactions with healthcare professionals, healthcare organizations, patients, and other customers; business consulting services in the nature of auditing of pharmaceutical and medical device manufacturer interactions with and payments to healthcare professionals, healthcare organizations, patients, and other customers; business consulting services in the nature of business investigation support for internally and externally raised complaints made against a pharmaceutical or medical device manufacturer related to potential violations of their company policy or code, industry code, or law or regulation; transparency reporting regulatory submission management, namely, assisting others in preparing and filing regulatory submissions in the field of transparency reporting for pharmaceutical, biotechnology and medical device companies; business consulting services, namely, tracking and monitoring regulatory requirements for others in the field of life sciences, pharmaceuticals, biotechnology, and medical devices for regulatory compliance; business consulting services, namely, compliance program gap analysis for life sciences, pharmaceutical, biotechnology, and medical device companies
MedPro Systems LLC
100 Stierli Court, Mt. Arlington, NEW JERSEY, UNITED STATES, 07856
100 Stierli Court, Mt. Arlington, NEW JERSEY, UNITED STATES, 07856
CONNECTID
Filed on Jun 02, 2023
Construction consultancy; construction of civil engineering structures; construction administration services, namely, construction project management services, construction supervision, and construction management
Walter P. Moore and Associates, Inc.
Suite 1100,1301 McKinney, Houston, TEXAS, UNITED STATES, 77010
Suite 1100,1301 McKinney, Houston, TEXAS, UNITED STATES, 77010
INSTREAM EARLYPAY
Filed on Jun 02, 2023
Financial advisory services, namely, expert structuring and analysis in finance and implementation of asset finance solutions; financial structuring services; financial investment services, namely, administering issuance, modelling, underwriting, and distribution of trade assets; financial risk management; Investment services, namely, asset structuring, origination, underwriting, pricing, acquisition, trading, monitoring, and compliance of trade assets
Katsumi Inc.
600 Edgewater Drive, Unit 304, Dunedin, FLORIDA, UNITED STATES, 34698
600 Edgewater Drive, Unit 304, Dunedin, FLORIDA, UNITED STATES, 34698
ZUBARR
Filed on Jun 02, 2023
Battery chargers for mobile phones; Chargers for smartphones; Holders adapted for mobile telephones and smartphones; Mobile phone chargers; Smartphone battery chargers; USB charging ports; Wireless battery chargers; Wireless chargers; Wireless charging stands for smartphones
Guangzhou Binyi Technology Co., Ltd
N1173, Room 103, No. 137,Heguang Road, Tianhe District, Guangzhou, CHINA, 510665
N1173, Room 103, No. 137,Heguang Road, Tianhe District, Guangzhou, CHINA, 510665
NBNC NUESTRA BELLEZA NACIONAL CUBA
Filed on Jun 02, 2023
I will be selling advertisement for our pageant magazine
NBNC_CUBA_OFICIAL
16223 SW 108th Ct. Miami, FL 33157, Miami, FLORIDA, UNITED STATES, 33157
16223 SW 108th Ct. Miami, FL 33157, Miami, FLORIDA, UNITED STATES, 33157
CANISIUS UNIVERSITY
Filed on Jun 02, 2023
Education services in the nature of courses at the university level
The Canisius College of Buffalo, N.Y.
Lyons Hall 212,2001 Main Street, Buffalo, NEW YORK, UNITED STATES, 14208
Lyons Hall 212,2001 Main Street, Buffalo, NEW YORK, UNITED STATES, 14208
NATURADE IMPACT
Filed on Jun 02, 2023
Powdered nutritional supplement drink mix
Prevention, LLC
2030 Main Street, Suite 630, Irvine, CALIFORNIA, UNITED STATES, 92614
2030 Main Street, Suite 630, Irvine, CALIFORNIA, UNITED STATES, 92614
MILITARY DICK
Filed on Jun 02, 2023
Downloadable photographs in the field of adult oriented subject matter; Downloadable video recordings featuring adult oriented subject matter
Charged Media LLC
111 NE 1st St FL 2, Miami, FLORIDA, UNITED STATES, 33132
111 NE 1st St FL 2, Miami, FLORIDA, UNITED STATES, 33132
BAUSCH
Filed on Jun 02, 2023
ARTICULATING PAPERS FOR ARTICULATION AND OCCLUSION CONTROL IN DENTISTRY
DR. JEAN BAUSCH GMBH & CO. KG
OSKAR SCHINDLER STR. 4, COLOGNE GERMANY 50769
OSKAR SCHINDLER STR. 4, COLOGNE GERMANY 50769
EVERYTHING YOU KNOW ABOUT DINOSAURS IS PREHISTORIC.
Filed on Jun 02, 2023
The trademark is a tag line for a touring museum experience, a large format film and an app all using the same information about dinosaurs
Weiss, David
8016 Naylor Avenue, Los Angeles, CALIFORNIA, UNITED STATES, 90045
8016 Naylor Avenue, Los Angeles, CALIFORNIA, UNITED STATES, 90045